Citric Acid Cycle
- The citric acid cycle also known as the tricarboxylic acid cycle or the krebs cycle was discovered in 1937, through intensive efforts to understand the pathways of aerobic respiration.
- The cycle is responsible for the oxidisation of about two thirds of the cells carbon compounds. It generates the products carbon dioxid as a waste product and NADH. The NADH complexes carry the high energy electrons required for the electron transport chain.
- Once the pyruvate is converted into the actyl CoA it enters the citric acid acyle were it undergoes a series of reactions.
STEP 1
- The acetyl CoA enters the cycle were it is first converted into citrate (6C).The enzyme removes a proton (H+) from the CH group on the acetyl CoA leaving a negatively charged CH-. The negatively charged CH forms a bound to a carbonyl carbon of oxaloacetate. The subsequental loss by hydrolysis of CoA drives the final step to form the conversion to citrate.
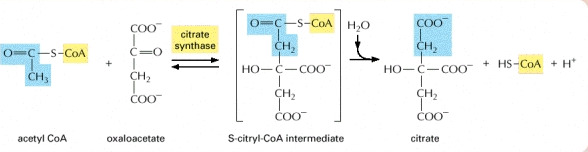
Image courtesy of www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A163#A304
STEP 2
- In the seconded step the citrate is converted into isocitrate. An isomerisation reaction causes the movement of the hydroxyl group from one carbon atom to its neighbour. This reaction requires the addition and then the removal of water
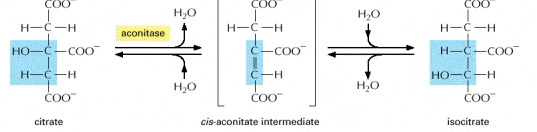
Image courtesy of www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A163#A304
STEP 3
- The isocitrate is converted into a-ketoglutarate in this step. This is the first of the four oxidation steps that occur in the cycle.The generation of a carbonyl group from the conversion of the carbon atom carrying the hydroxyl group occurs. The product results in an unstable intermediate and loses CO2 (carbon dioxide) while still bound to the enzyme.
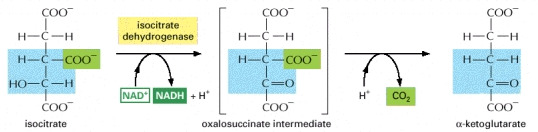
Image courtesy of www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A163#A304
STEP 4
- In this step succiny-CoA is formed through the oxidation of the a-ketoglutarate. The a-ketoglutarate dehdanse complex catalyzes the oxidation step which results in the generation of a NADH, CO2 and a high energy thioester bond to CoA in the –ketoglurate complex.
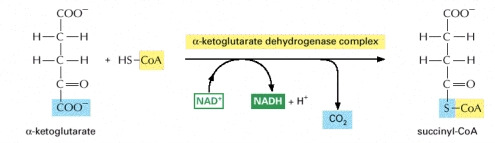
Image courtesy of www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A163#A304
STEP 5
- In this step the succiny-CoA is then converted into succinate by a displacement reaction. A phosphate molecule displaces the CoA to form a high-energy phosphate linkage to succinate.This phosphate is then passed to GDP to form GTP.
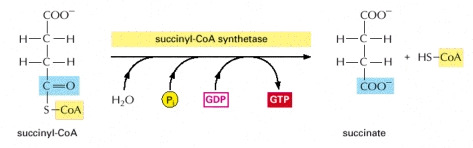
Image courtesy of www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A163#A304
STEP 6
- The third oxidation step occurs at this stage, resulting in the product fumrate. FAD removes two hydrogen atoms from succinate.
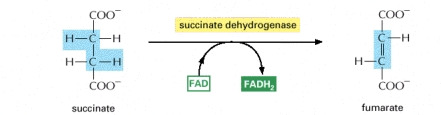
Image courtesy of www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A163#A304
STEP 7
- The fumarate is then converted into malate by the addition of water. In which a hydroxyl group becomes placed next to the carbonyl compound.
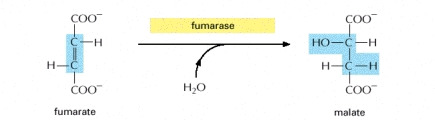
Image courtesy of www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A163#A304
STEP 8
- The last of the four oxidation steps occurs at this stage of the cycle. The oxaloacetate is regenerated from the malate. This occurs by converting the carbon carrying the hydroxyl group, into a carbonyl group. The regeneration of the oxaloacetate is then required for step one.
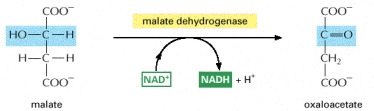
Image courtesy of www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A163#A304
SUMMARY
- Overall the reaction of the CoA with the oxaloacetate initiates the cycle by producing citric acid.
- In each turn of the cycle 3 NADH molecules, 1 molecule of GTP and 1 molecule of FADH2 are generated.
- Two CO2 molecules are also produced as waste products.
- The electron carriers NADH and FADH2 then go on to transfer the electrons gained from the oxidation reactions in the citric acid cycle, to the electron transport chain.
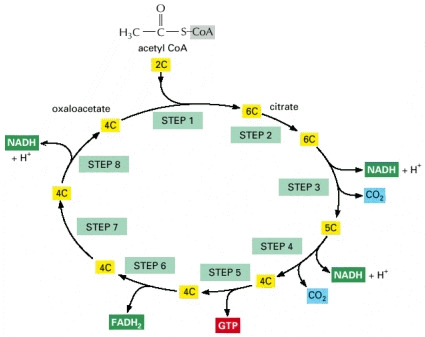
Image courtesy of www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=mboc4&part=A163#A304